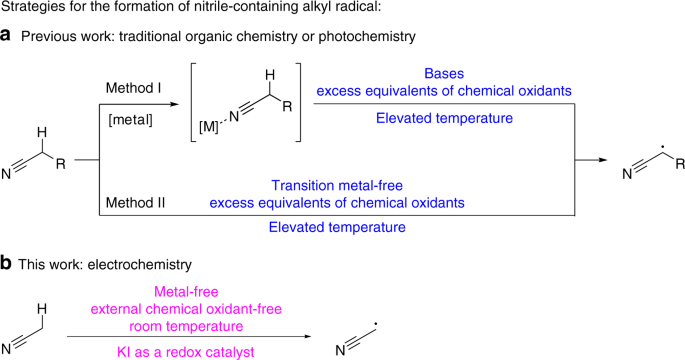
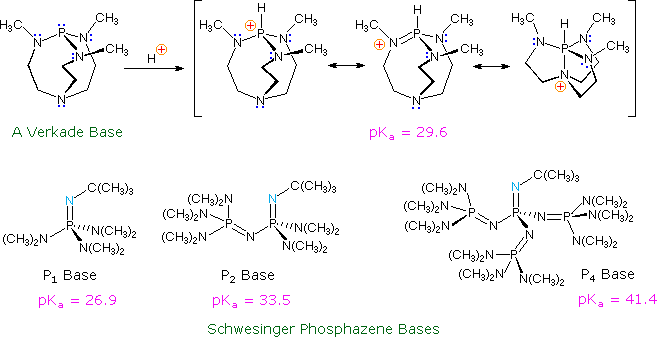
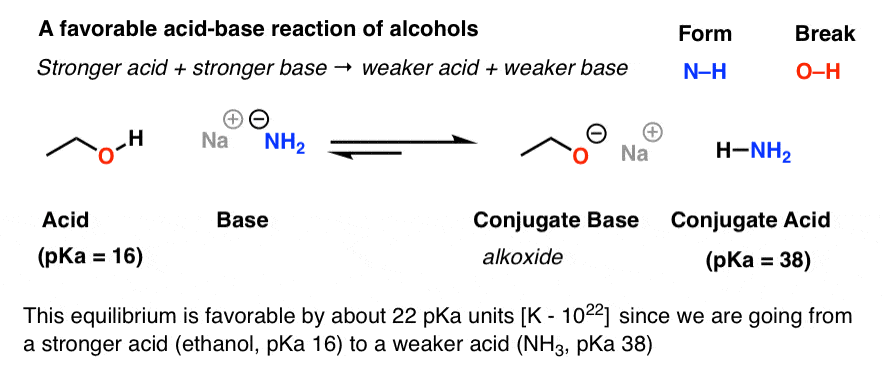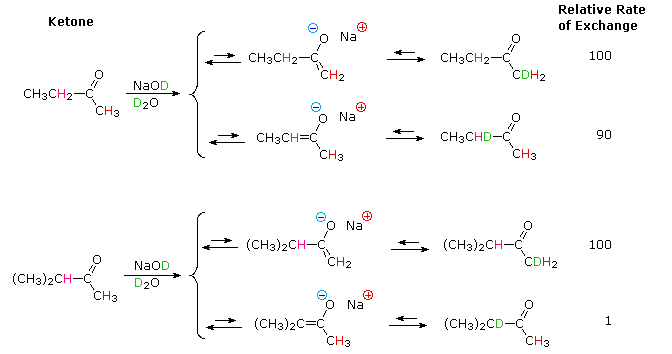Schematic representation of acetamide formation (A) from acetonitrile... | Download Scientific Diagram

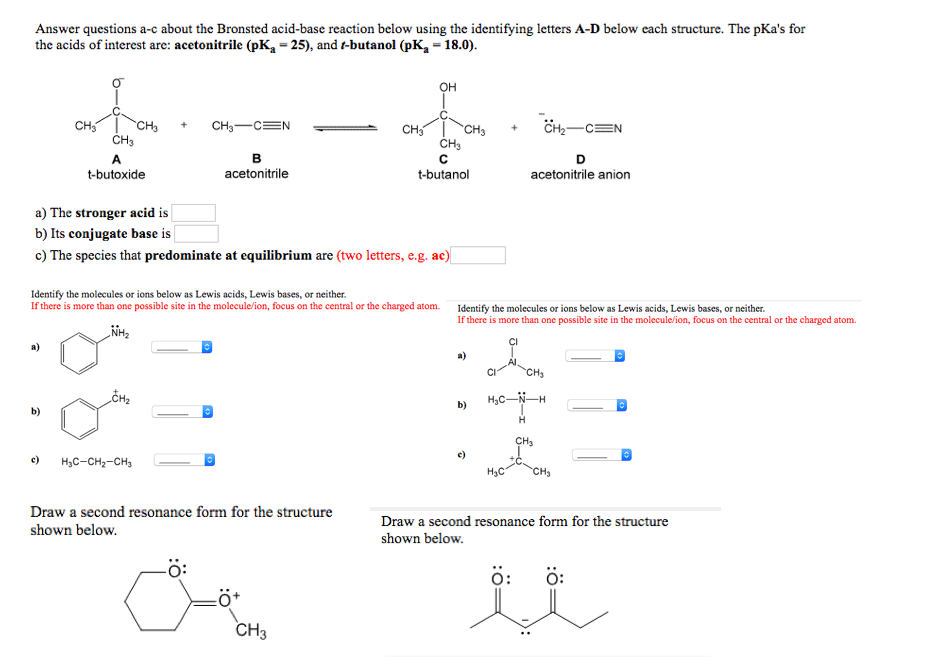
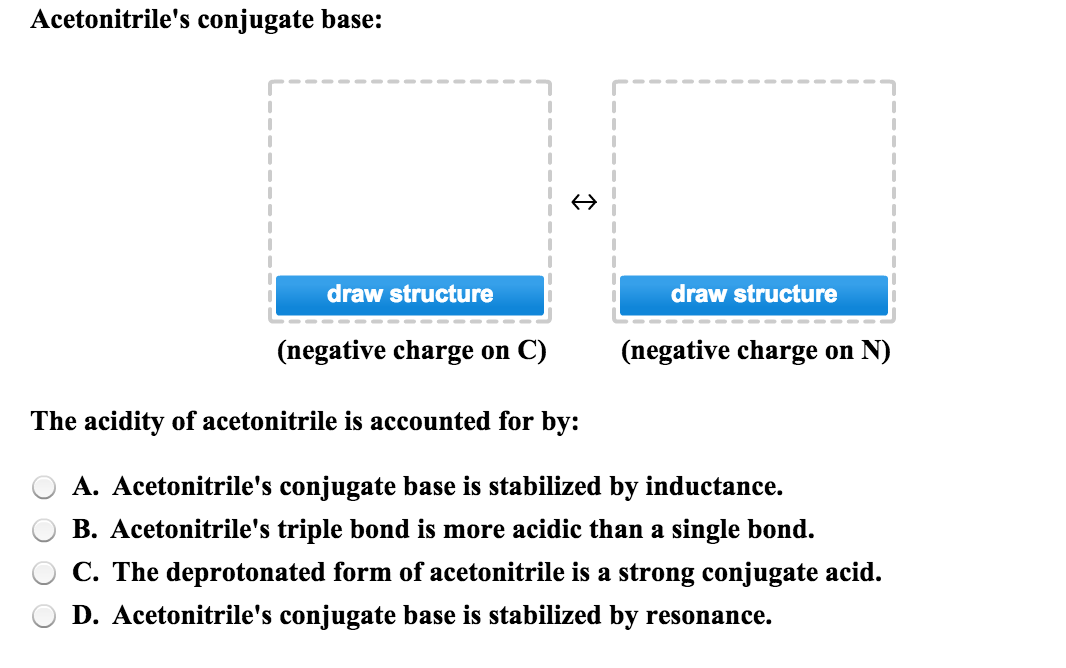
Acetonitrile (CH3CN) has a pKa of 25, making it more acidic than many other compounds having only C - H bonds. Draw structures for acetonitrile and its conjugate base. Use resonance structures
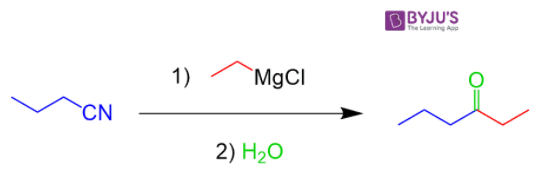
Acetonitrile (C2H3N) - Structure, Properties of Acetonitrile, Molecular Weight & Uses of Acetonitrile

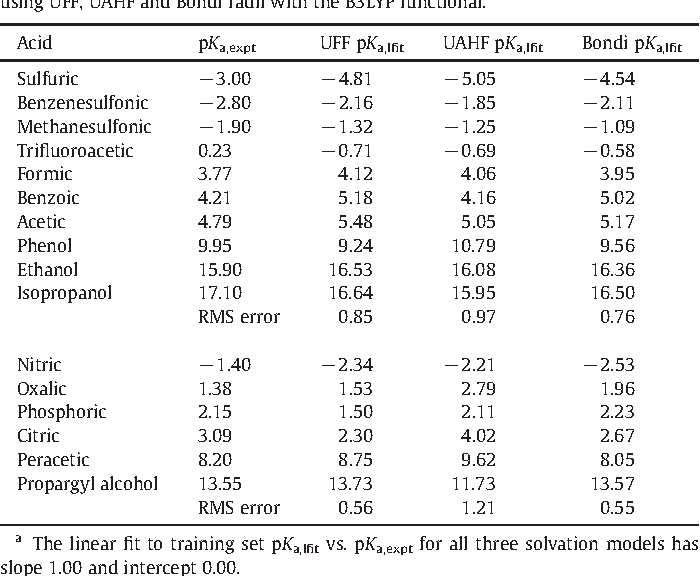
Strengths of Acids in Acetonitrile - Kütt - 2021 - European Journal of Organic Chemistry - Wiley Online Library
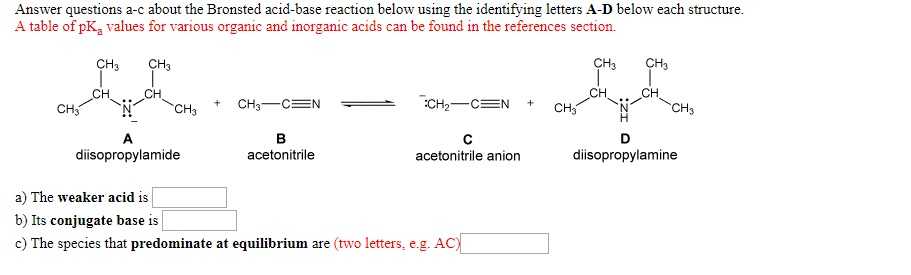
![SOLVED: [Review Topics] [References] Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each struc A table of pKa values for various organic and inorganic acids SOLVED: [Review Topics] [References] Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each struc A table of pKa values for various organic and inorganic acids](https://cdn.numerade.com/ask_images/cf87821c9d3a451e9683ce641a1833a9.jpg)
SOLVED: [Review Topics] [References] Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each struc A table of pKa values for various organic and inorganic acids

Strengths of Acids in Acetonitrile - Kütt - 2021 - European Journal of Organic Chemistry - Wiley Online Library
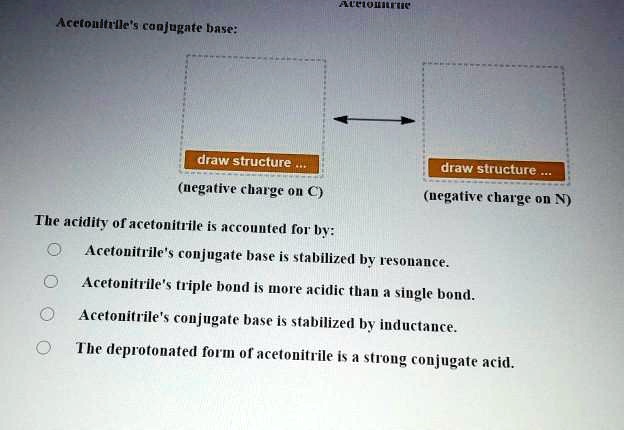
SOLVED: Acetoulttlle' conjugate base: draw structure draw structure (uegative charge o C) (uegative charge ' on N The acidity of acetonitrile is acconuted [or by: Acetonitrile's conjugate base is stabilized by resonance:

Influence of acid-base properties of the support on the catalytic performances of Pt-based catalysts in a gas-phase hydrogenation of acetonitrile - ScienceDirect